For important risk and use information, please see the full Prescribing Information, including Boxed Warning.
Results in Treatment-naïve and Pretreated PAH Patients from the PATENT-1 Study
Video title
Efficacy data in PATENT-1 were only measured for the Adempas 2.5 mg and placebo arms. The data in the treatment-naïve and pretreated subgroup analyses should be considered exploratory and were not designed to detect statistically significant differences in these subgroups.
PATENT-1 Study Design
PIVOTAL TRIAL IN ADULT PATIENTS WITH PAH (WHO GROUP 1)1
Randomized, double-blind, multinational, placebo-controlled, 12-week, phase 3 study

Primary endpoint1
- Change in 6MWD from baseline to Week 12
Baseline characteristics1
- Mean age: 51 years (approximately 80% female)
- PAH etiologies: idiopathic (61%), associated with connective tissue disease (25%), congenital heart disease (8%), portal hypertension (3%), familial (2%), or anorexigen or amphetamine use (1%)
- Mean 6MWD was 363 m
- Concomitant medications: oral anticoagulants, diuretics, digitalis, calcium channel blockers, and oxygen were allowed
- Patient population: 50% treatment naïve, 44% pretreated with an endothelin receptor antagonist (ERA), and 6% pretreated with a prostacyclin analog (PCA)
- The majority of patients had WHO FC II (42%) or III (54%) at baseline
- Patients with systolic blood pressure <95 mm Hg were excluded
6MWD = 6-minute walk distance; m = meters; mPAP = mean pulmonary arterial pressure; PAH = pulmonary arterial hypertension; PATENT-1 = Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator Trial; PVR = pulmonary vascular resistance; WHO FC = World Health Organization Functional Class.
PATENT-1 Results
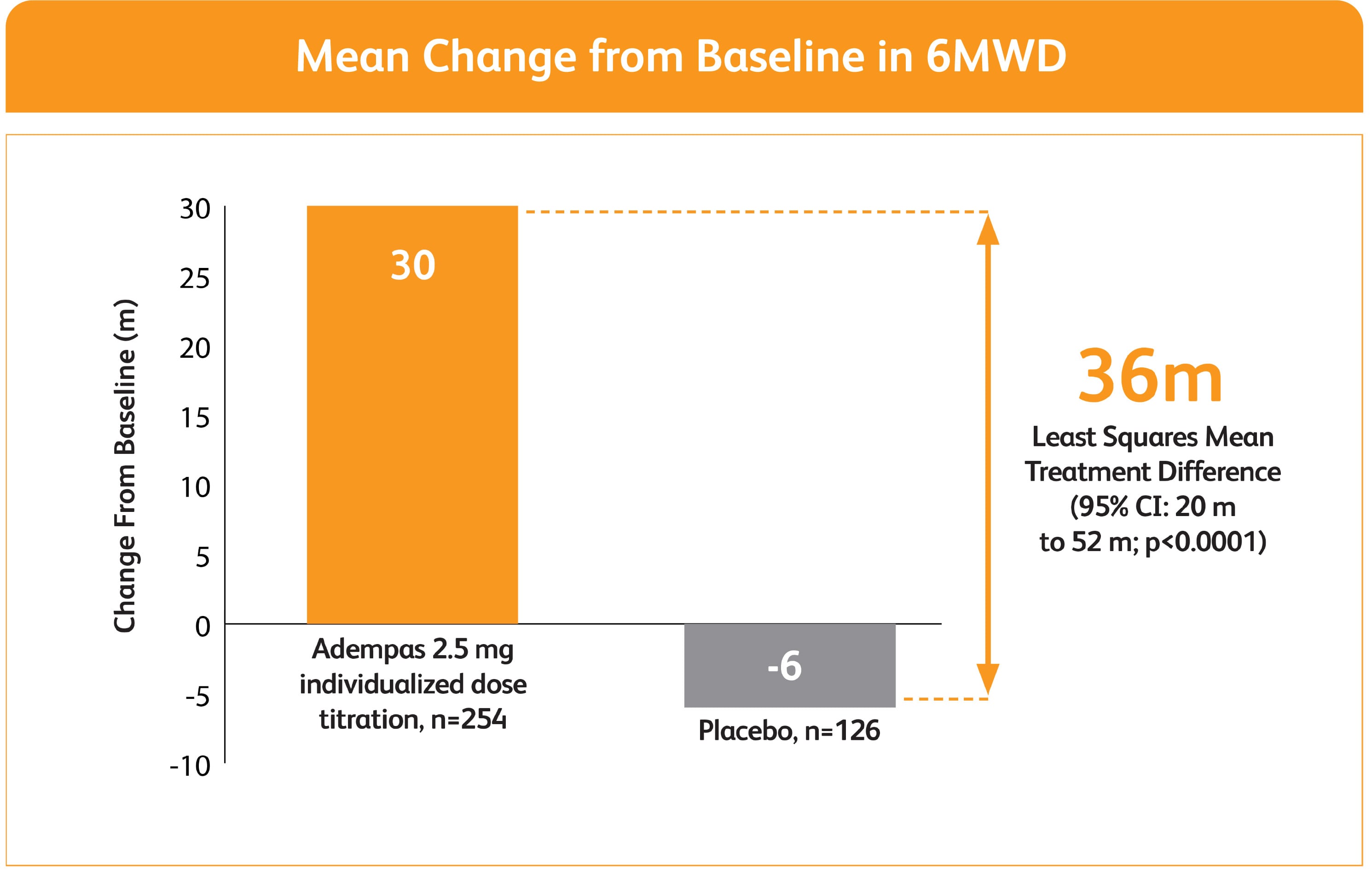
IMPROVEMENT IN 6MWD WAS OBSERVED AT 2 WEEKS AND CONTINUED THROUGH 12 WEEKS1
SIGNIFICANT IMPROVEMENT IN 6MWD AT WEEK 12
Adempas can be used as monotherapy or in combination
- In WHO FC II and III
- As monotherapy
- In combination with endothelin receptor antagonists (ERAs)
- In combination with prostacyclin analogs (PCAs)
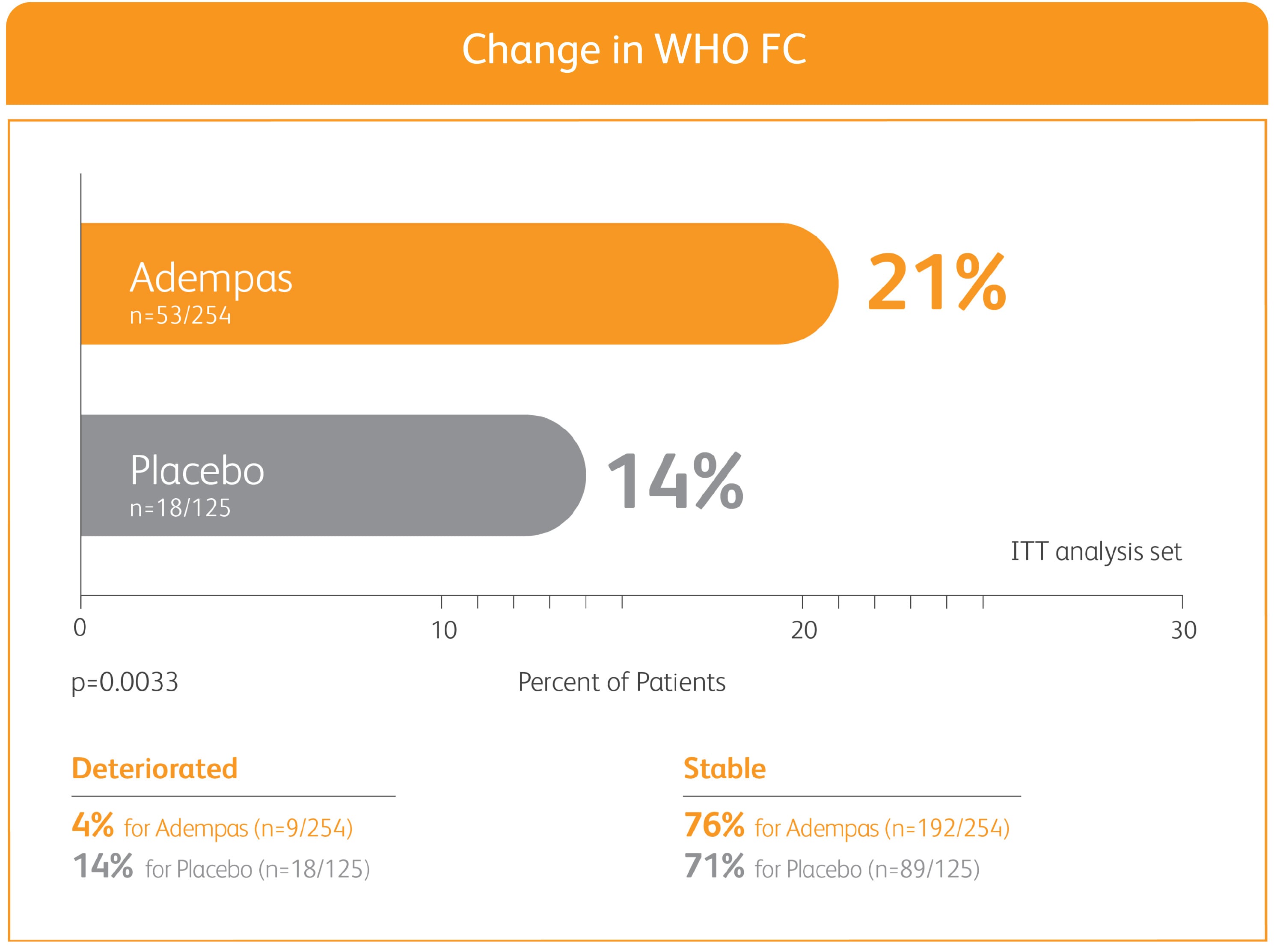
50% MORE PATIENTS IMPROVED WHO FC ON ADEMPAS VS PLACEBO
CHANGE IN WHO FC IN THE 12-WEEK PATENT-1 STUDY
ITT = intention-to-treat; WHO FC = World Health Organization Functional Class.
ADEMPAS IMPROVED PVR AND NT-proBNP AT WEEK 12*
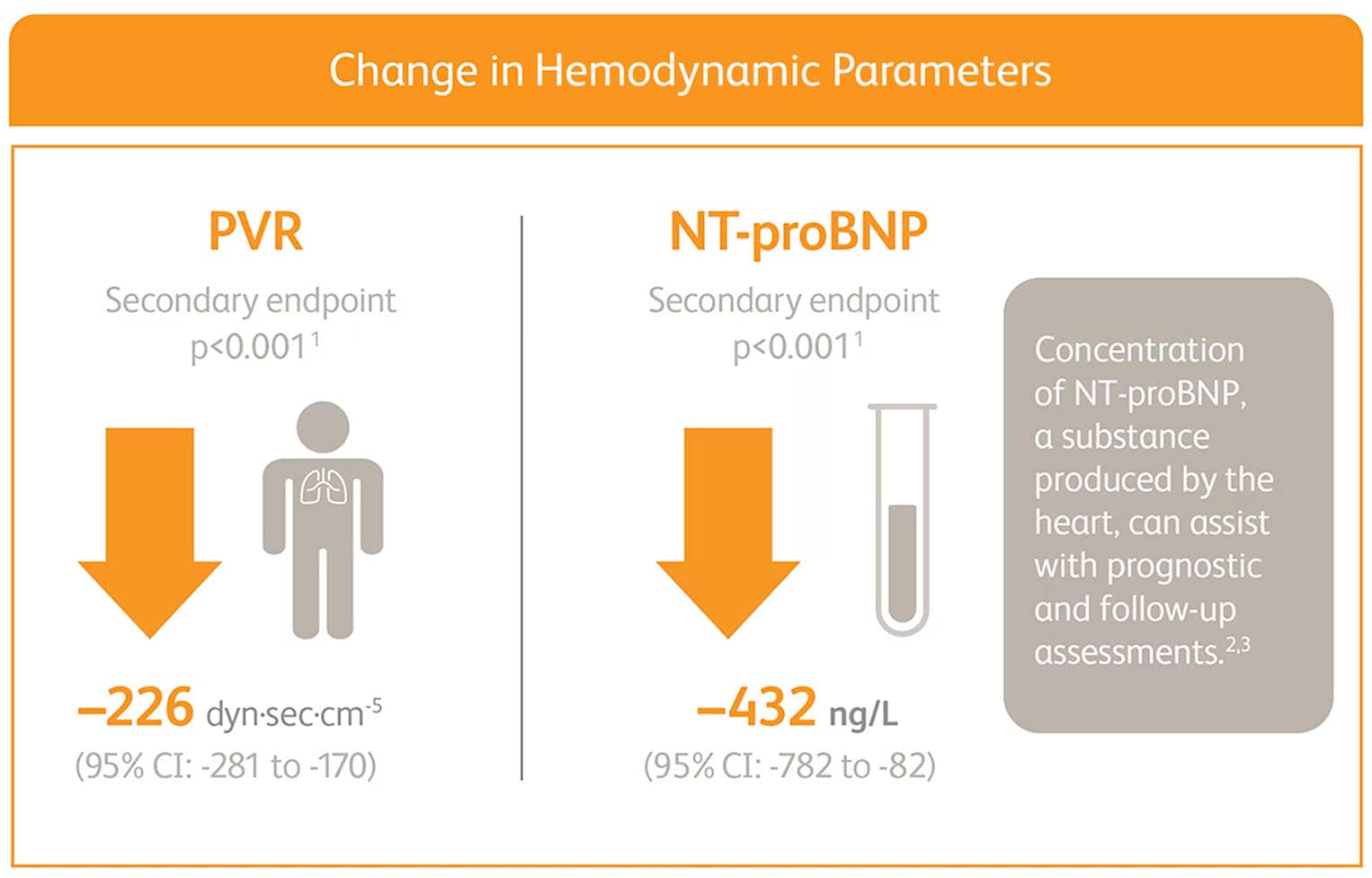
Right heart catheterization was performed at the beginning and end of the study period in 339 patients.
*Placebo-adjusted mean change from baseline.
NT-proBNP = N-terminal pro-brain natriuretic peptide.
CLINICAL WORSENING EVENTS UP TO 12 WEEKS
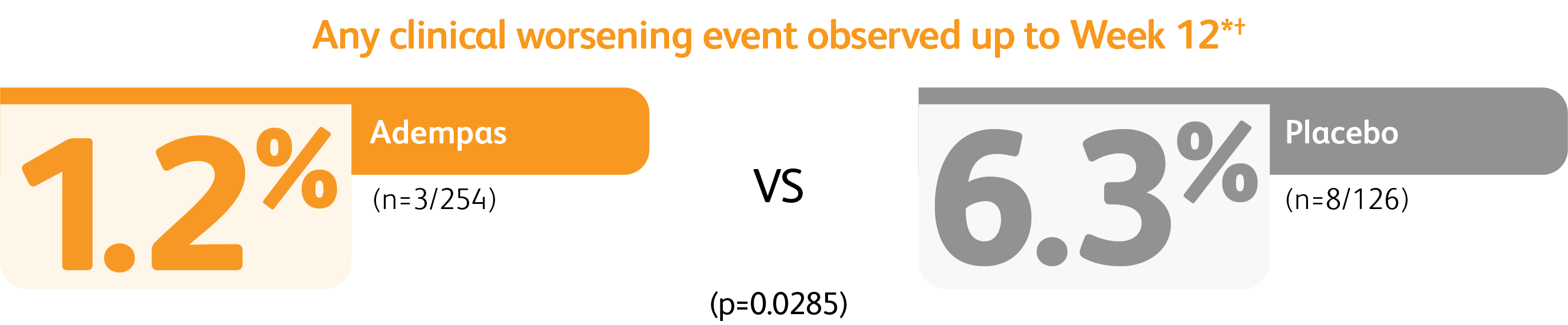
*Time to clinical worsening was a combined endpoint defined as death (all-cause mortality), heart/lung transplantation, atrial septostomy, hospitalization due to persistent worsening of PH, start of new PAH-specific treatment, persistent decrease in 6MWD, and persistent worsening of WHO Functional Class.
†Patients may have had more than one event of clinical worsening.
PATENT-1 Study Design
PIVOTAL TRIAL IN ADULT PATIENTS WITH PAH (WHO GROUP 1)1,2
Randomized, double-blind, multinational, placebo-controlled, 12-week phase 3 study
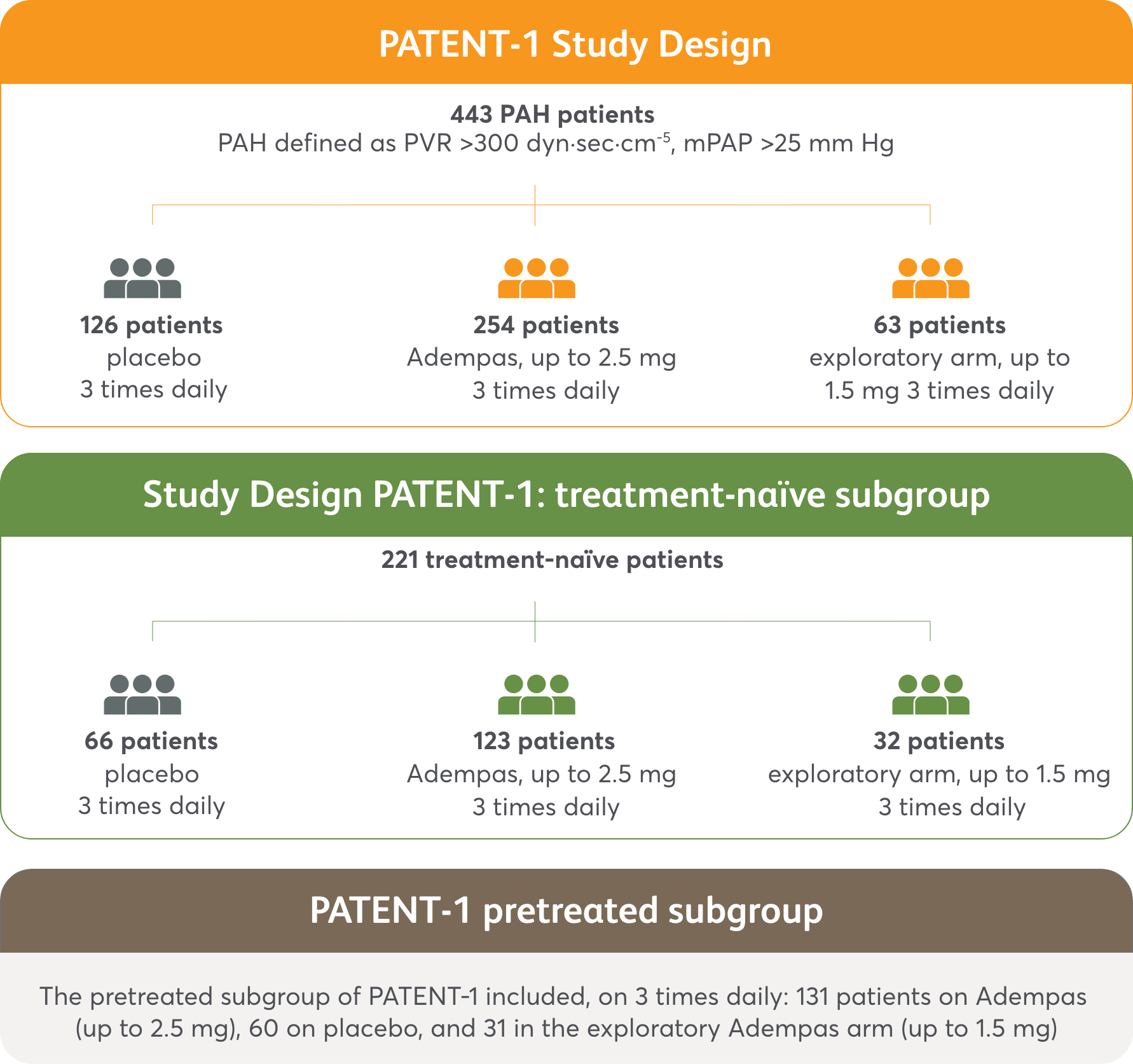
PATENT-1 primary endpoint
- Change in 6MWD from baseline to Week 12
Secondary endpoints included changes from baseline to Week 12 in2:
- WHO FC
- Time to clinical worsening event
- PVR and NT-proBNP
Efficacy data were only measured for the Adempas 2.5 mg and placebo arms.
PATENT-1 Baseline Patient Characteristics3
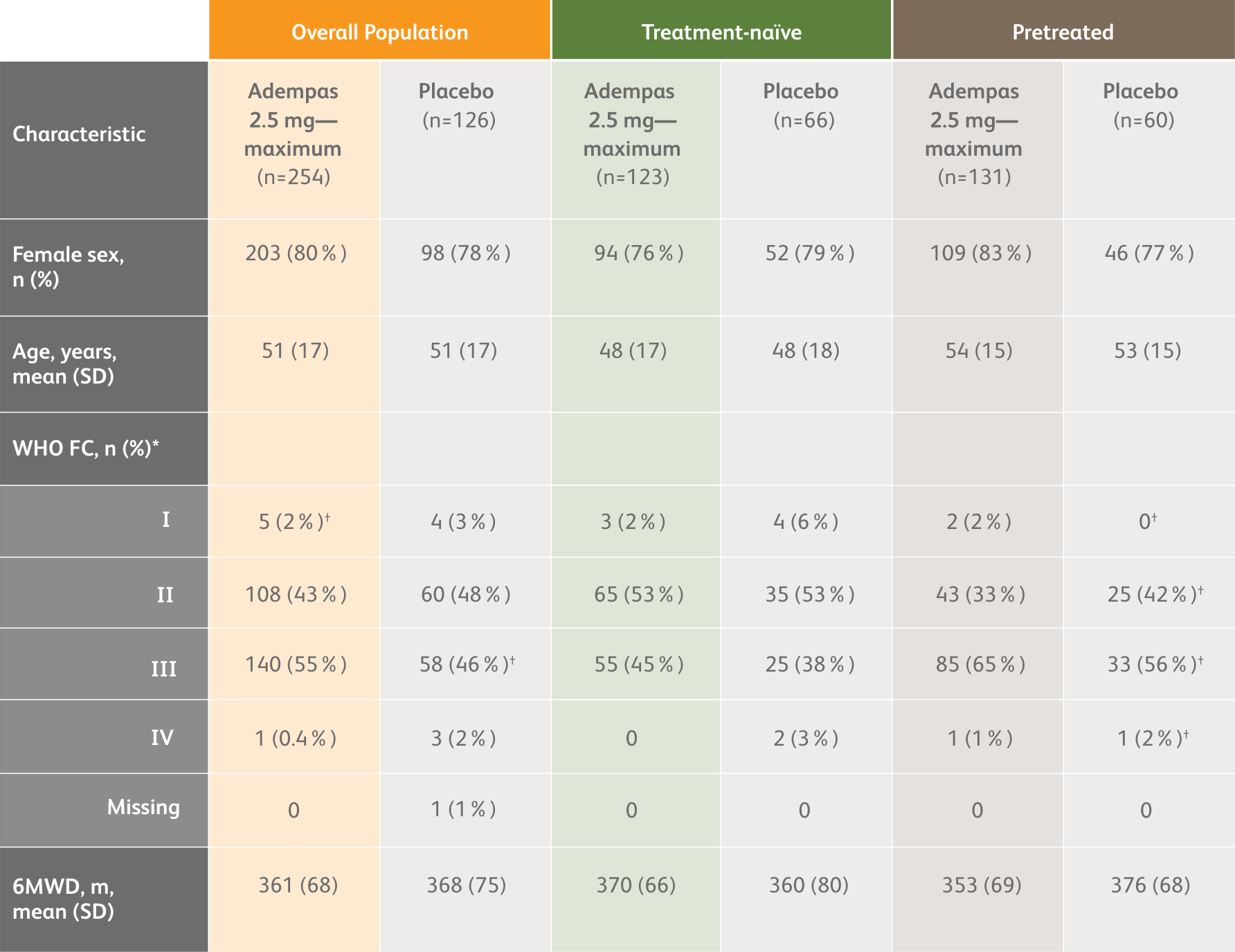
*Data may not add up to 100% owing to rounding.
†n=59.
PATENT-1 Results
6MWD AT WEEK 12 BASED ON PATIENT CHARACTERISTICS1
Mean Treatment Difference in Change From Baseline to Week 12* in 6MWD by Prespecified Subgroups
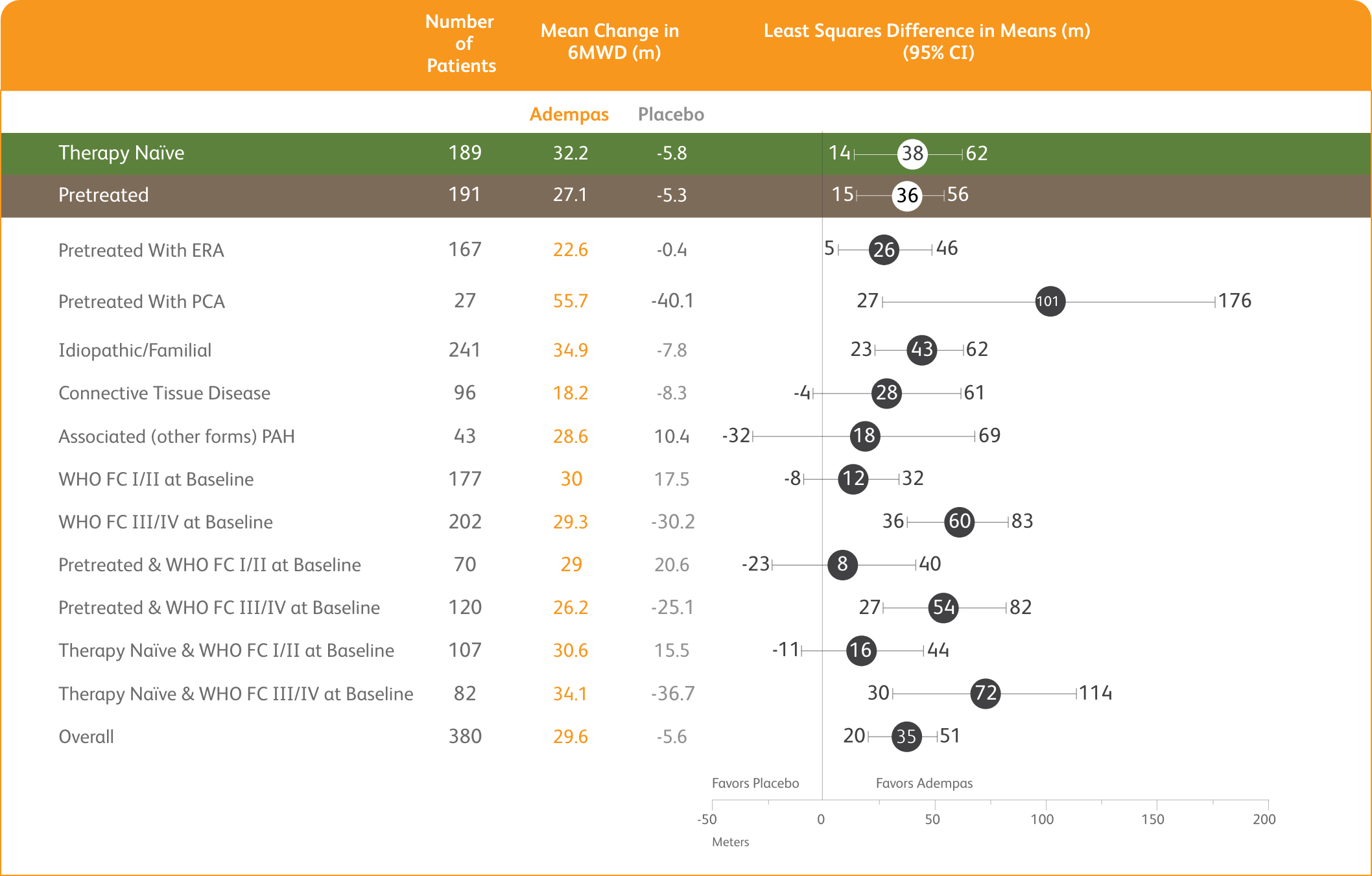
*Week 12/last observation until Week 12.
PATENT-1 (n=443): Improvement in 6MWD was observed at 2 weeks and continued through 12 weeks2
Primary Endpoint: Mean Change from Baseline in 6MWD
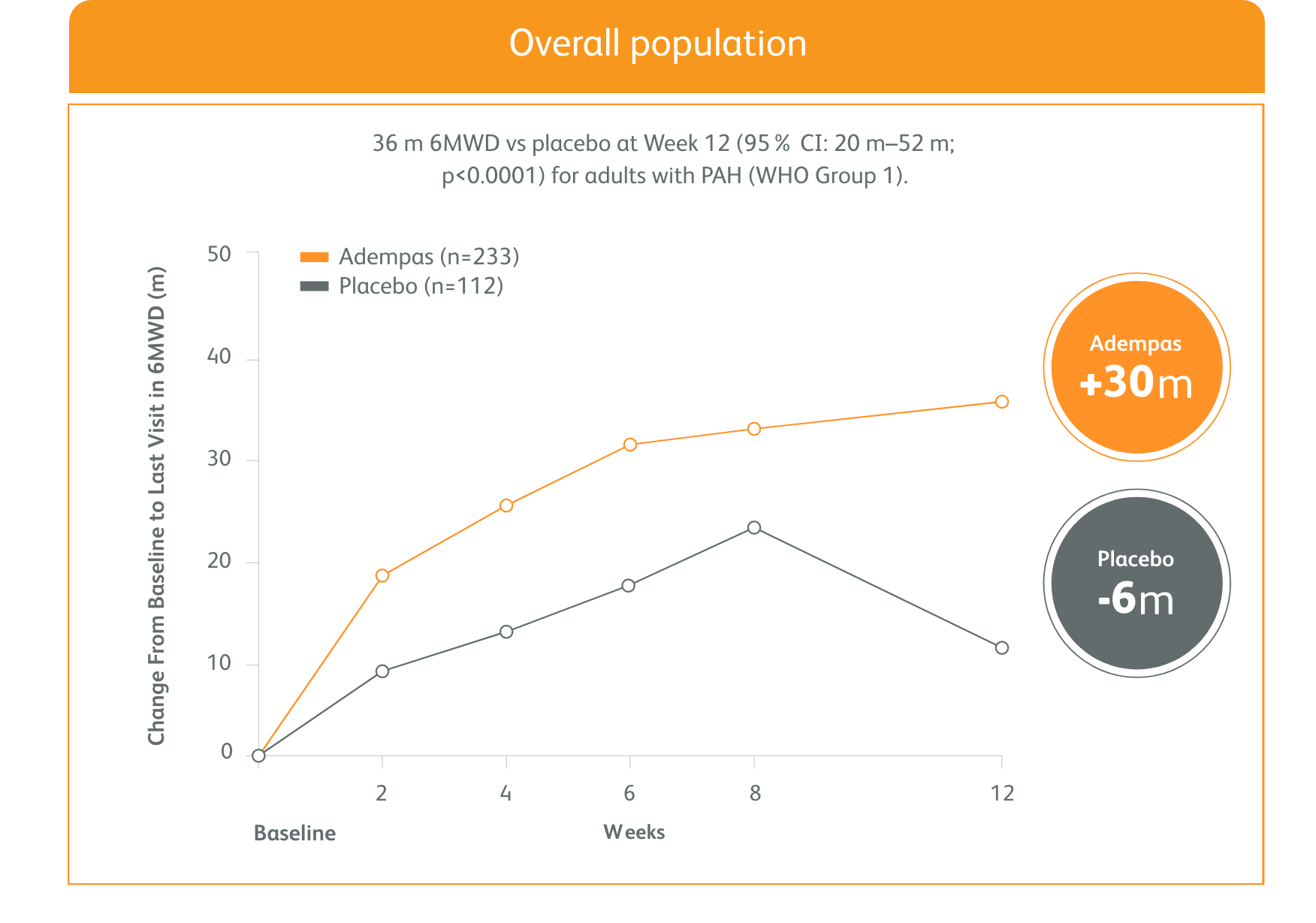
Exploratory post hoc subgroup analyses
PATENT-1 Subgroup Analysis: Change in 6MWD from baseline to Week 121
MEAN CHANGE FROM BASELINE IN 6MWD
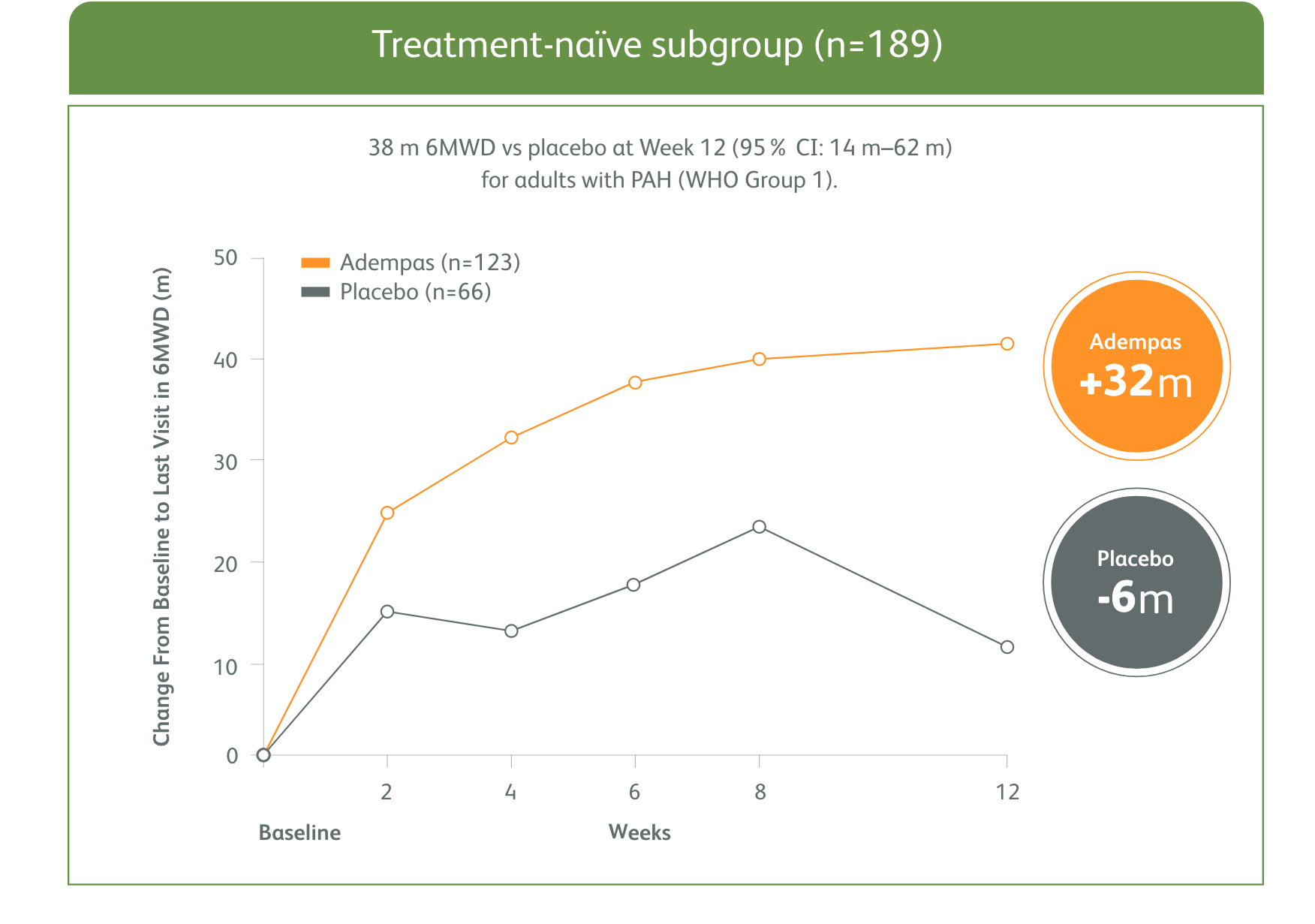
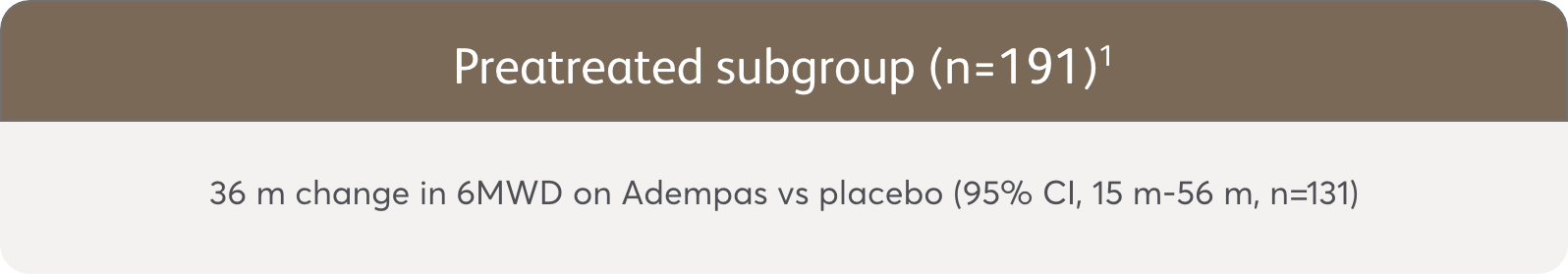
The data in this analysis should be considered exploratory and not designed to detect statistically significant differences in these subgroups.
PATENT-1 (n=443): 50% more patients improved WHO FC vs placebo
CHANGE IN WHO FC
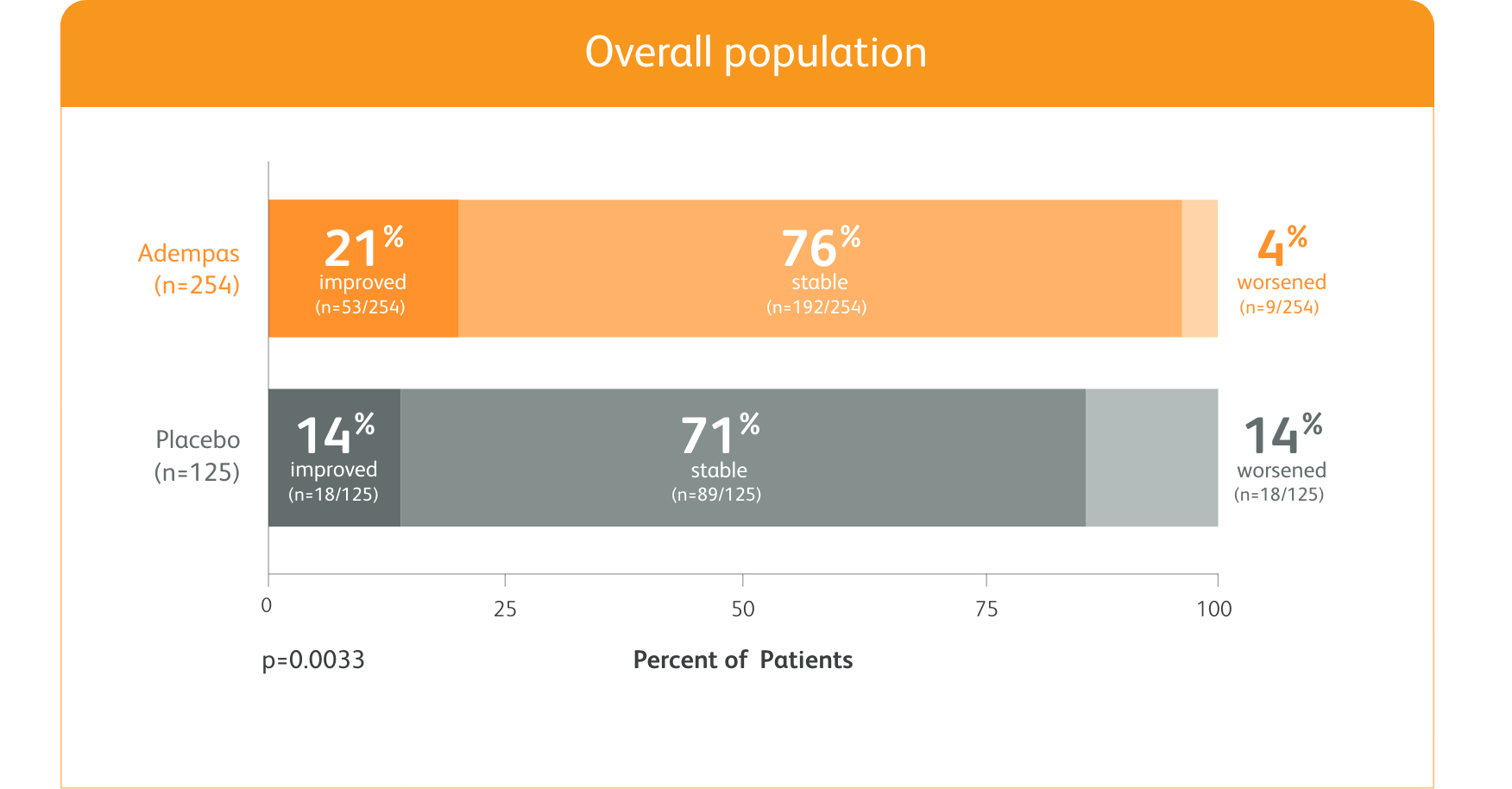
Exploratory post hoc subgroup analyses
PATENT-1 Subgroup Analysis: Change in WHO FC from baseline to Week 121
Change from baseline in WHO FC

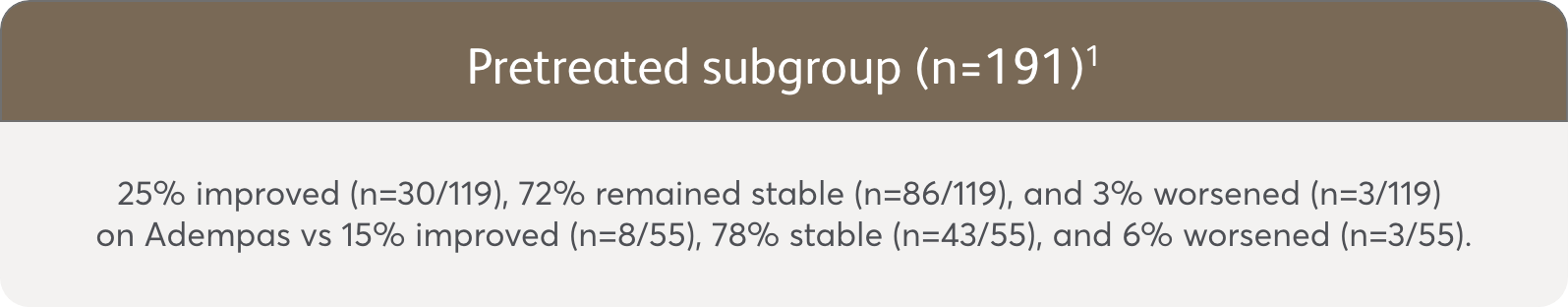
The data in this analysis should be considered exploratory and not designed to detect statistically significant differences in these subgroups.
PATENT-1 (n=443): Improved PVR and NT-proBNP at Week 122*
Change in hemodynamic parameters
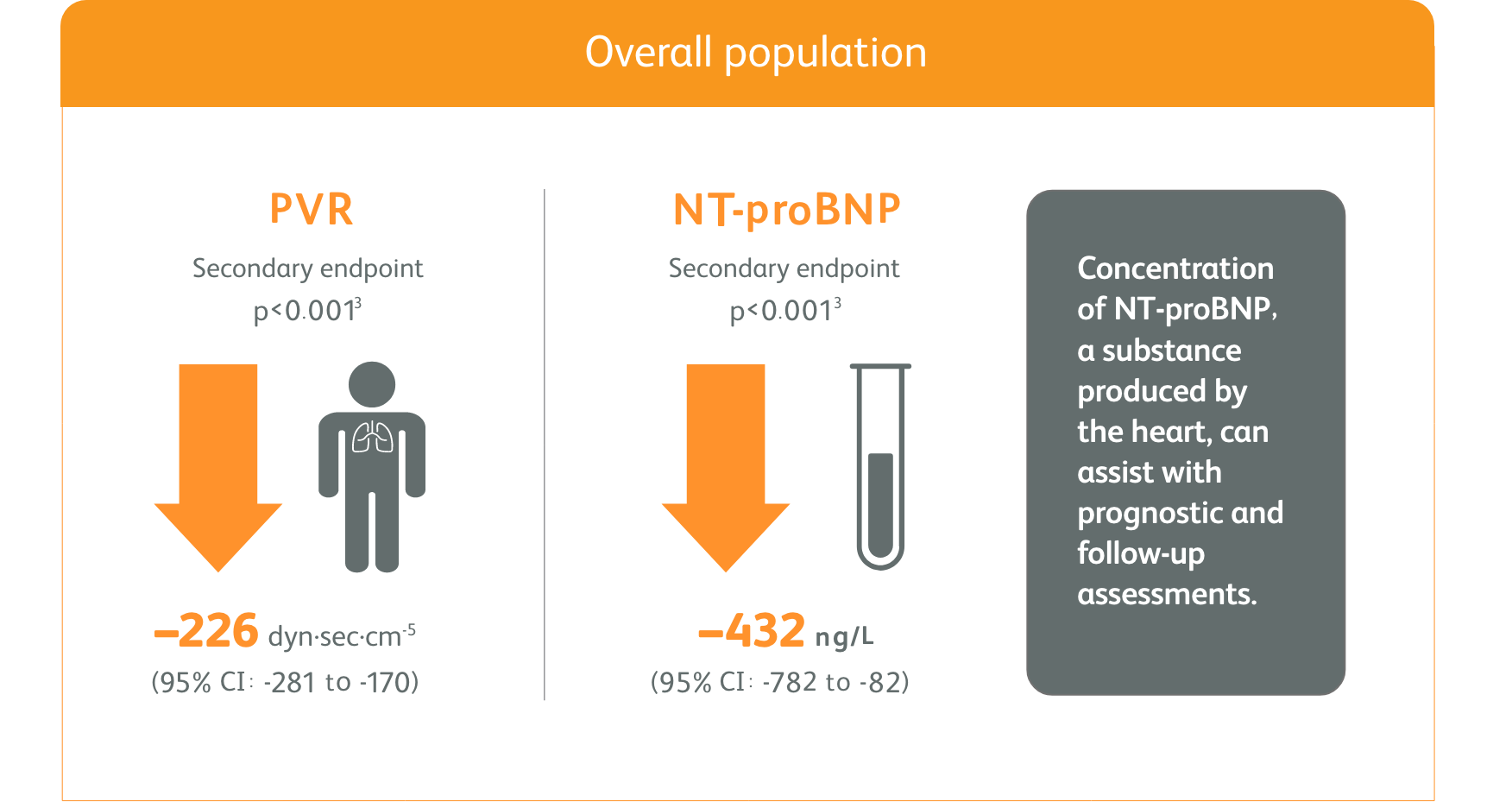
*Placebo-adjusted mean change from baseline.
- Right heart catheterization was performed at the beginning and end of the study period in 339 patients.
Exploratory post hoc subgroup analyses
Clinical worsening events up to 12 weeks
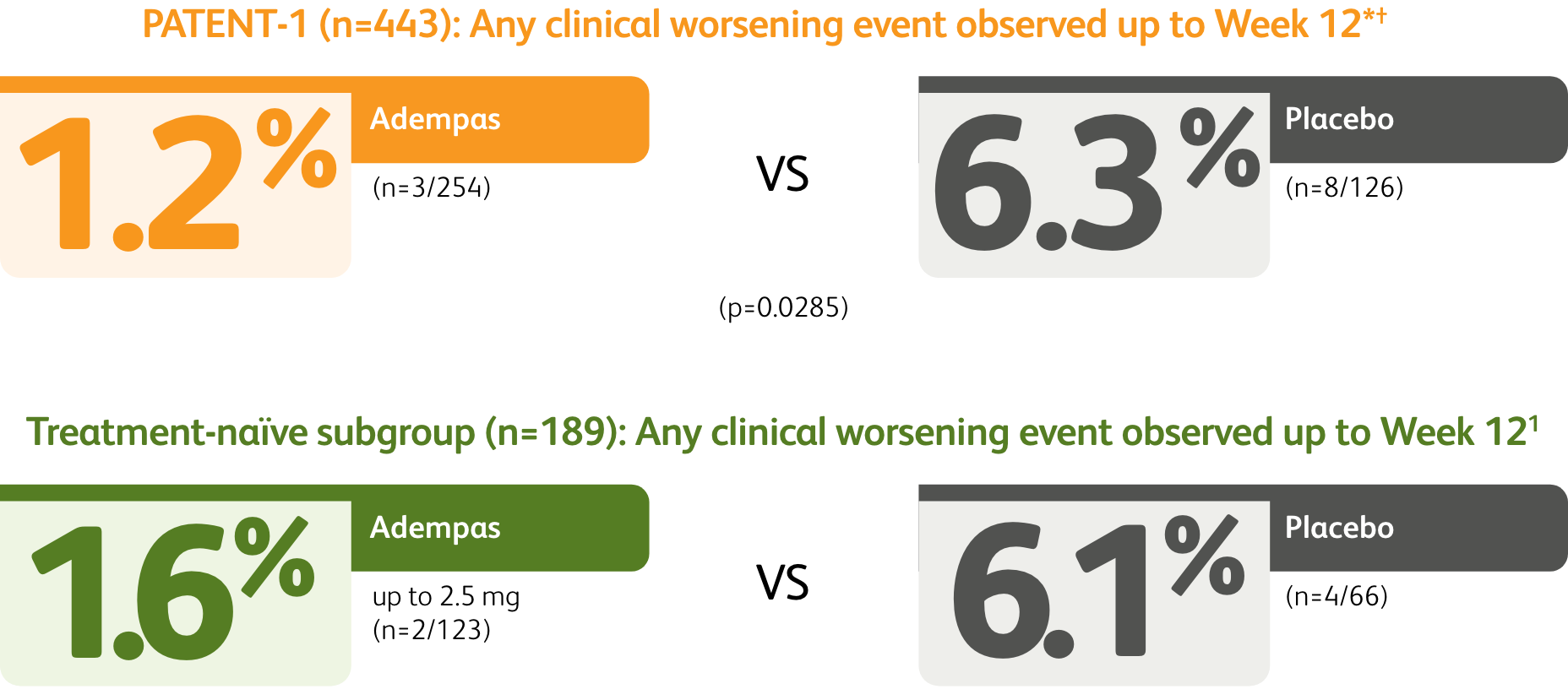

The data in this analysis should be considered exploratory and not designed to detect statistically significant differences in subgroups.
*Time to clinical worsening was a combined endpoint defined as death (all-cause mortality), heart/lung transplantation, atrial septostomy, hospitalization due to persistent worsening of pulmonary hypertension, start of new PAH-specific treatment, persistent decrease in 6MWD, and persistent worsening of WHO FC.
†Patients may have had more than one event of clinical worsening.
Exploratory post hoc subgroup analyses
PATENT-1 Subgroup Analysis: Change in PVR and NT-proBNP from baseline to Week 121
MEAN CHANGE FROM BASELINE IN PVR AND NT-proBNP
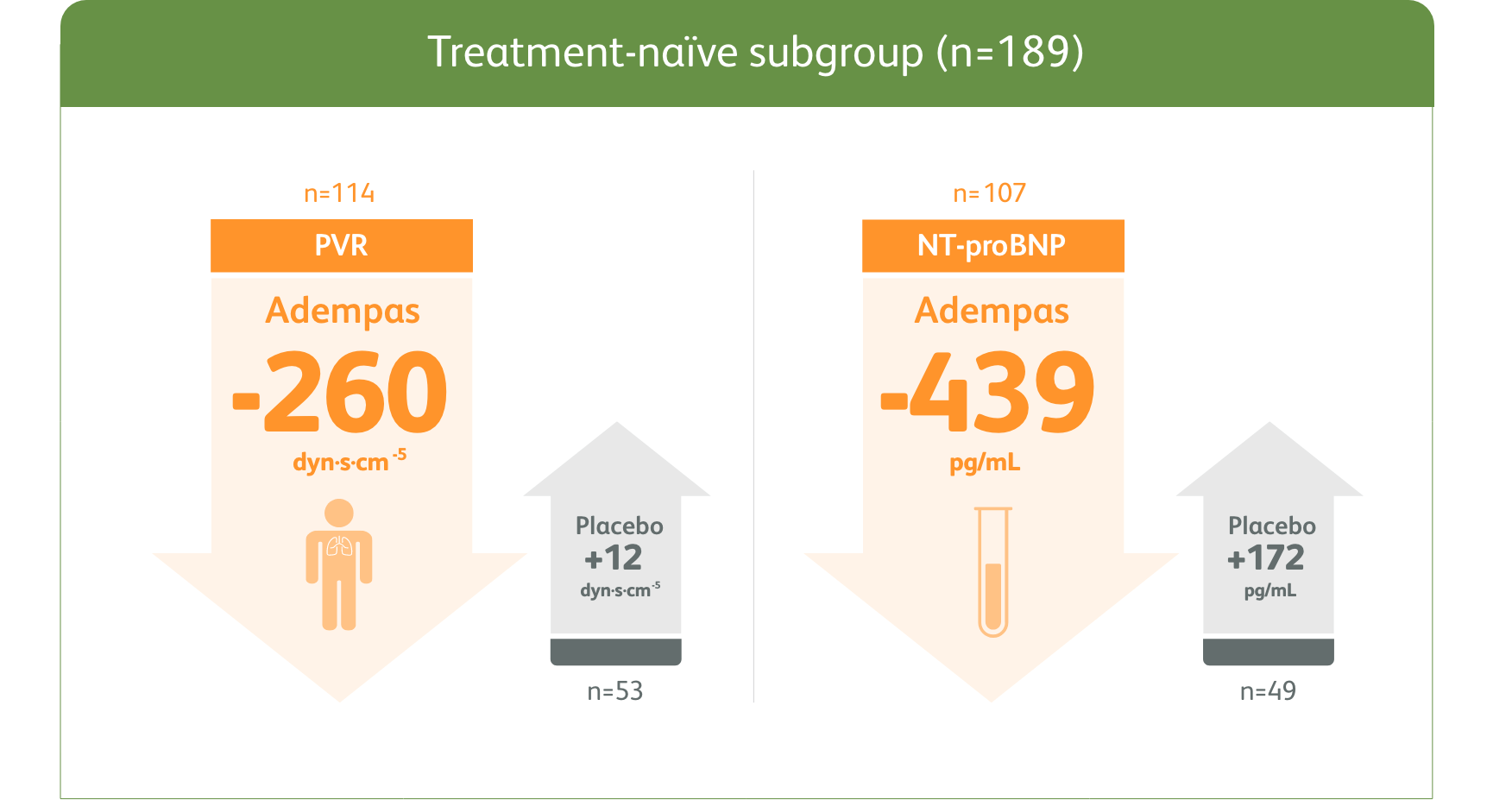

The data in this analysis should be considered exploratory and not designed to detect statistically significant differences in these subgroups.
6MWD = 6-minute walk distance; ERA = endothelin receptor antagonist; m = meters; mPAP = mean pulmonary arterial pressure; mm Hg = millimeters mercury; NT-proBNP = N-terminal pro-brain natriuretic peptide; PAH = pulmonary arterial hypertension; PATENT-1 = Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator Trial; PCA = prostacyclin analogues; PVR = pulmonary vascular resistance; SD = standard deviation; WHO FC = World Health Organization Functional Class.
WARNING: EMBRYO-FETAL TOXICITY
Do not administer Adempas (riociguat) tablets to a pregnant female because it may cause fetal harm.
Females of reproductive potential: Exclude pregnancy before the start of treatment, monthly during treatment, and one month after stopping treatment. To prevent pregnancy, females of reproductive potential must use effective forms of contraception during treatment and for one month after stopping treatment.
For all female patients, Adempas is available only through a restricted program called the Adempas Risk Evaluation and Mitigation Strategy (REMS) Program.
Adempas is contraindicated in:
- Pregnancy. Based on data from animal reproduction studies, Adempas may cause fetal harm when administered to a pregnant woman and is contraindicated in females who are pregnant. Adempas was consistently shown to have teratogenic effects when administered to animals. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Co-administration with nitrates or nitric oxide donors (such as amyl nitrite) in any form.
- Concomitant administration with specific phosphodiesterase (PDE)-5 inhibitors (such as sildenafil, tadalafil, or vardenafil) or nonspecific PDE inhibitors (such as dipyridamole or theophylline) is contraindicated. Do not administer within 24 hours of sildenafil. Do not administer 24 hours before or within 48 hours after tadalafil.
- Patients with concomitant use of other soluble guanylate cyclase (sGC) stimulators.
- Patients with Pulmonary Hypertension associated with Idiopathic Interstitial Pneumonias (PH-IIP).
Embryo-Fetal Toxicity. Based on data from animal reproduction studies, Adempas may cause embryo-fetal toxicity when administered to a pregnant female and is contraindicated in females who are pregnant. Advise females of reproductive potential of the potential risk to a fetus. Obtain a pregnancy test before the start of treatment, monthly during treatment, and for one month after stopping treatment. Advise females of reproductive potential to use effective contraception during treatment with Adempas and for at least one month after the last dose.
For females, Adempas is only available through a restricted program under the Adempas REMS Program.
Adempas REMS Program. Females can only receive Adempas through the Adempas REMS Program, a restricted distribution program.
Important requirements of the Adempas REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- All females, regardless of reproductive potential, must enroll in the Adempas REMS Program prior to initiating Adempas. Male patients are not enrolled in the Adempas REMS Program.
- Female patients of reproductive potential must comply with the pregnancy testing and contraception requirements.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive Adempas.
Further information, including a list of certified pharmacies, is available at www.AdempasREMS.com or 1-855-4ADEMPAS.
Hypotension. Adempas reduces blood pressure. Consider the potential for symptomatic hypotension or ischemia in patients with hypovolemia, severe left ventricular outflow obstruction, resting hypotension, autonomic dysfunction, or concomitant treatment with antihypertensives or strong CYP and P-gp/BCRP inhibitors. Consider a dose reduction if patient develops signs or symptoms of hypotension.
Bleeding. In the placebo-controlled clinical trials, serious bleeding occurred in 2.4% of patients taking Adempas compared to 0% of placebo patients. Serious hemoptysis occurred in 5 (1%) patients taking Adempas compared to 0 placebo patients, including one event with fatal outcome. Serious hemorrhagic events also included 2 patients with vaginal hemorrhage, 2 with catheter-site hemorrhage, and 1 each with subdural hematoma, hematemesis, and intra-abdominal hemorrhage.
Pulmonary Veno-Occlusive Disease. Pulmonary vasodilators may significantly worsen the cardiovascular status of patients with pulmonary veno-occlusive disease (PVOD). Therefore, administration of Adempas to such patients is not recommended. Should signs of pulmonary edema occur, the possibility of associated PVOD should be considered and if confirmed, discontinue treatment with Adempas.
The most common adverse reactions occurring more frequently (≥3%) on Adempas than placebo were headache (27% vs 18%), dyspepsia/gastritis (21% vs 8%), dizziness (20% vs 13%), nausea (14% vs 11%), diarrhea (12% vs 8%), hypotension (10% vs 4%), vomiting (10% vs 7%), anemia (7% vs 2%), gastroesophageal reflux disease (5% vs 2%), and constipation (5% vs 1%).
Other events that were seen more frequently in Adempas compared to placebo and potentially related to treatment were palpitations, nasal congestion, epistaxis, dysphagia, abdominal distension, and peripheral edema.
- Adempas (riociguat) is indicated for the treatment of adults with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH) (WHO Group 4) after surgical treatment or inoperable CTEPH to improve exercise capacity and WHO functional class.
- Adempas is indicated for the treatment of adults with pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise capacity, improve WHO functional class and to delay clinical worsening.*
Efficacy was shown in patients on Adempas monotherapy or in combination with endothelin receptor antagonists or prostanoids. Studies establishing effectiveness included predominantly patients with WHO functional class II–III and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (25%).
*Time to clinical worsening was a combined endpoint defined as death (all-cause mortality), heart/lung transplantation, atrial septostomy, hospitalization due to persistent worsening of pulmonary hypertension, start of new PAH-specific treatment, persistent decrease in 6MWD, and persistent worsening of WHO functional class.
For important risk and use information, please see the full Prescribing Information, including Boxed Warning.
References:
- Ghofrani H-A, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330-340.
- Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67-119.
- McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D73-D81.
References:
- Data on file. Clinical study report No. A62510. Bayer HealthCare Pharmaceuticals, 2012.
- Ghofrani H-A, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330-340.
- Galiè N, Grimminger F, Grünig E, et al. Comparison of hemodynamic parameters in treatment-naïve and pre-treated patients with pulmonary arterial hypertension in the randomized phase III PATENT-1 study. J Heart Lung Transplant. 2017;36(5):509-519.



